
Dr
Zouridakis Marios
(independent research group since Sep. 2021)
The research group of Structural Neurobiology belongs to the Laboratory of Molecular Neurobiology and Immunology. The conducted research deals mainly with structural studies of proteins that are pharmacological targets.
The research focus of the group is the elucidation of the X-ray crystal structures of various proteins of pharmacological interest and of the structures of their complexes with ligands (i.e. chemical molecules, peptides) and/or antibodies that could serve as potential drugs for the treatment of related diseases. Moreover, of great interest is to decipher the functional mechanisms of the studied proteins through structure-guided mutagenesis and functional assays. Also, in collaboration with other members of the Laboratory of Molecular Neurobiology and Immunology, some of the recombinant proteins used for structural studies serve as antigens for the development of diagnostic assays.
Main research interests of the group:
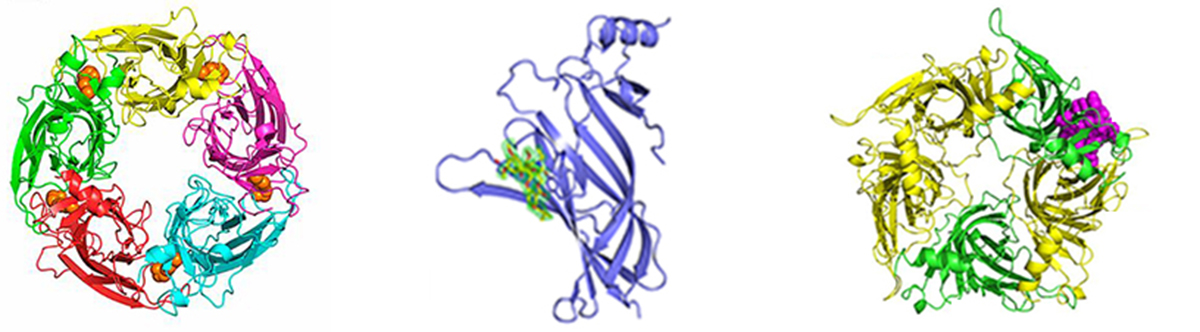
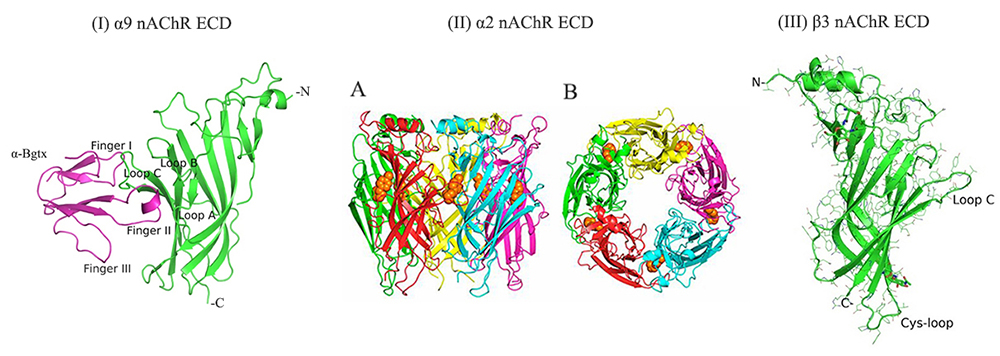
Structural studies of ligand-gated ion channels. A major target for elucidation of its 3D-structure is the human neuronal nicotinic acetylcholine receptor (nAChR). Neuronal nAChRs regulate neuronal excitability and neurotransmitter release and exist in various pentameric subtypes formed by combinations between eleven subunits (α2-α7, α9, α10, β2-β4), each with distinct pharmacological and electrophysiological properties. They are responsible for nicotine addiction and are implicated in several neurological diseases and disorders (e.g. Alzheimer’s and Parkinson’s, autism, schizophrenia). Interestingly, neuronal nAChRs (e.g. α7, α9, α10) exist also in a variety of cells such as immune cells, breast and lung cells, keratinocytes, etc, and have been found to play important roles in inflammation and in various cancer types. Elucidation of the structure of neuronal nAChRs in their Apo and ligand-bound forms is needed for the design of subtype-specific drugs and for understanding their functional mechanisms. Also, of interest is the elucidation of the crystal structures of the human muscle nAChR (the autoantigen in myasthenia gravis disease) with novel neurotoxins and Fab domains of monoclonal antibodies.
Structural studies of the ACE2 receptor. Driven by the COVID-19 pandemic, the group has initiated efforts to elucidate the X-ray crystal structures of the human Angiotensin Converting Enzyme 2 receptor (ACE2), which is the established entry point of the SARS-CoV2 coronavirus to the cells, in its complexes with the receptor-binding domains (RBDs) of the original Wuhan strain and with the emerged variants. In addition, the group is interested in structural studies of ACE2 and ACE receptors with novel inhibitors in order to provide the structural basis for the design of novel ligands (inhibitors or activators) of the Renin-Angiotensin-Aldosterone System (RAAS) for the regulation of blood pressure and fluid balance. Moreover, the group provides the recombinantly expressed RBD and ACE2 proteins to other members of the lab for the development of Ab-detecting assays for vaccinated and recovered individuals.
Structural studies of Tyrosinases. Tyrosinase is a multi-copper enzyme which is widely distributed in different organisms and plays an important role in the melanogenesis and enzymatic browning. Hyperpigmentation in human skin as well as browning of fruits, vegetables and fungi are common undesirable effects related to tyrosinases. Our group is interested in studying structurally and functionally both the human and mushroom tyrosinases. We anticipate that our studies will reveal the unique structural features of these enzymes and will assist to the development of novel, potent and selective inhibitors. Those molecules can be attractive in cosmetics and medicinal industries as depigmentation agents as well as in food and agriculture industries as anti-browning compounds.
Functional studies of receptors and enzymes. The group is interested in complementing its structural findings with functional studies. For nAChRs, the group studies their pharmacological properties and the effect of novel ligands with radio-ligand binding experiments and with two-electrode voltage clamp electrophysiology, while for the ACE2 enzyme the group studies the effect of potentially novel inhibitors (or activators) with classical kinetic studies. Also, of great interest is the discovery of autoantibodies against neuronal nAChRs in several diseases of the nervous system such as schizophrenia, bipolar disorders, epilepsy, etc, in collaboration with the other research group of the lab and with external collaborators.
Prof. Dimitris Georgiadis, NKUA, Athens
Prof. Georgios Spyroulias, University of Patras
Assoc. Prof. Kostas Bethanis, Agricultural University of Athens
Assoc. Prof. Efstratios Stratikos, NKUA, Athens
Prof. Georgios Vougioukalakis, NKUA, Athens
Dr Spyros Zografos, National Hellenic Research Foundation
Dr Uwe Maskos, Institut Pasteur, Paris
Dr Friederike Jonsson, Institut Pasteur, Paris
Prof. Chris Ulens, KU, Leuven, Belgium
Prof. Victor Tsetlin, Russian Academy of Sciences, Russia
Prof. Michael McIntosh, University of Utah, USA
- Institut Pasteur, Paris, Transversal Research programs (PTR): Role of human auto-antibodies against nicotinic acetylcholine receptors in psychiatric disease PTR no 695 – 2023 Anti-NIC (Role of M.Z.: P.I. for the Hellenic Pasteur Institute) 01/01/2024-31/12/2025
- General Secretariat for Research and Innovation under the action “National research network to elucidate the genetic basis of Alzheimer’s and Parkinson’s neurodegenerative diseases, detect reliable biomarkers, and develop innovative computational technologies and therapeutic strategies on the basis of precision medicine” TAA TAEDR-0535850—BrainPrecision, European Union, NextGenerationEU, National Recovery and Resilience Plan Greece 2.0. (Role of M.Z.: Work packages leader) 15/05/2023-14/09/2025
- Hellenic Foundation for Research and Innovation (HFRI) Project, Structural and functional studies of nicotinic acetylcholine receptors, StruNic, code number 677, 2018-2022
- Stavros Niarchos Foundation, Development of innovative biological products 2016-2020
Ridgway H, Moore GJ, Gadanec LK, Zulli A, Apostolopoulos V, Hoffmann W, Węgrzyn K, Vassilaki N, Mpekoulis G, Zouridakis M, Giastas P, Vidali VP, Kelaidonis K, Matsoukas MT, Dimitriou M, Mavromoustakos T, Tsiodras S, Gorgoulis VG, Karakasiliotis I, Chasapis CT, Matsoukas JM. (2024) Novel benzimidazole angiotensin receptor blockers with anti-SARS-CoV-2 activity equipotent to that of nirmatrelvir: computational and enzymatic studies. Expert Opin Ther Targets 2024 May;28(5):437-459. doi: 10.1080/14728222.2024.2362675
Kokkorakis N, Zouridakis M, Gaitanou M. (2024) Mirk/Dyrk1B Kinase Inhibitors in Targeted Cancer Therapy. Pharmaceutics 2024 Apr 11;16(4):528. doi: 10.3390/pharmaceutics16040528
Darrau E, Jacquemet E, Pons S, Schlick L, Zouridakis M, Wu CL, Richard JR, Barau C, Le Corvoisier P, Yolken R, Tamouza R, Leboyer M, Maskos U. (2024) Serum autoantibodies against α7-nicotinic receptors in subgroups of patients with bipolar disorder or schizophrenia: clinical features and link with peripheral inflammation. Transl Psychiatry 2024 Mar 14;14(1):146. doi: 10.1038/s41398-024-02853-8
Giastas P, Papakyriakou A, Tsafaras G, Tzartos SJ & Zouridakis M. (2022) Structural Insights into the Role of β3 nAChR Subunit in the Activation of Nicotinic Receptors. Molecules. 2022;27(14):4642 doi: 10.3390/molecules27144642
Michail M, Zouvelou V, Belimezi M, Haroniti A, Zouridakis M & Zisimopoulou P. (2022) Analysis of nAChR Autoantibodies Against Extracellular Epitopes in MG Patients. Front Neurol. 2022;13:858998. doi: 10.3389/fneur.2022.858998.
Lebedev, D.S., Kryukova, E.V., Ivanov, I.A., Egorova, N.S., Timofeev, N.D., Spirova, E.N., Tufanova, E.Yu., Siniavin, A.E., Kudryavtsev, D.S., Kasheverov, I.E., Zouridakis, M., Katsarava, R., Zavradashvili, N., Iagorshvili, I., Tzartos, S.J., & Tsetlin, V.I. (2019) Oligoarginine Peptides, a New Family of Nicotinic Acetylcholine Receptor Inhibitors. Mol Pharmacol. 2019;96(5):664-673. doi:10.1124/mol.119.117713
Giastas P, Mpakali A, Papakyriakou A, Lelis A, Kokkala P, Neu M, Rowland P, Liddle J, Georgiadis D, Stratikos E. (2019) Mechanism for antigenic peptide selection by endoplasmic reticulum aminopeptidase 1. Proc Natl Acad Sci U S A. 2019 Dec 26;116(52):26709-26716. doi: 10.1073/pnas.1912070116.
Zouridakis, M., Papakyriakou, A., Ivanov, I.A., Kasheverov, I.E., Tsetlin, V., Tzartos, S., & Giastas, P. (2019) Crystal Structure of the Monomeric Extracellular Domain of α9 Nicotinic Receptor Subunit in Complex With α-Conotoxin RgIA: Molecular Dynamics Insights Into RgIA Binding to α9α10 Nicotinic Receptors. Front Pharmacol.2019;10:474. doi:10.3389/fphar.2019.00474
Giastas P, Andreou A, Papakyriakou A, Koutsioulis D, Balomenou S, Tzartos SJ, Bouriotis V, Eliopoulos EE. (2018)Structures of the Peptidoglycan N-Acetylglucosamine Deacetylase Bc1974 and Its Complexes with Zinc Metalloenzyme Inhibitors. Biochemistry. 2018 Feb 6;57(5):753-763. doi: 10.1021/acs.biochem.7b00919. Epub 2018 Jan 5. PMID: 29257674.
Kryukova, E. V., Ivanov, I. A., Lebedev, D. S., Spirova, E. N., Egorova, N. S., Zouridakis, M., Kasheverov, I. E., Tzartos, S. J., & Tsetlin, V. I. (2018) Orthosteric and/or Allosteric Binding of α-Conotoxins to Nicotinic Acetylcholine Receptors and Their Models. Mar Drugs. 2018;16(12):460. doi:10.3390/md16120460
Giastas P, Zouridakis M & Tzartos SJ¶ (2018) Understanding structure-function relationships of the human neuronal acetylcholine receptor: insights from the first crystal structures of neuronal subunits. Br J Pharmacol.2018;175(11):1880-1891. doi:10.1111/bph.13838
Vulfius CA, Kasheverov IE, Kryukova EV, Spirova EN, Shelukhina IV, Starkov VG, Andreeva TV, Faure G, Zouridakis M, Tsetlin VI, & Utkin YN (2017) Pancreatic and snake venom presynaptically active phospholipases A2 inhibit nicotinic acetylcholine receptors. PLoS One. 2017;12(10):e0186206. doi:10.1371/journal.pone.0186206
Lykhmus O, Koval L, Pastuhova D, Zouridakis M, Tzartos S, Komisarenko S, & Skok M (2016) The role of carbohydrate component of recombinant α7 nicotinic acetylcholine receptor extracellular domain in its immunogenicity and functional effects of resulting antibodies. Immunobiol. 2016;221(12):1355-1361. doi:10.1016/j.imbio.2016.07.012
Lykhmus O, Gergalova G, Zouridakis M, Tzartos S, Komisarenko S & Skok M (2015) Inflammation decreases the level of alpha7 nicotinic acetylcholine receptors in the brain mitochondria and makes them more susceptible to apoptosis induction. Int Immunopharmacol. 2015;29(1):148-151. doi:10.1016/j.intimp.2015.04.007
Lykhmus O, Voytenko L, Koval L, Mykhalskiy S, Kholin V, Peschana K, Zouridakis M, Tzartos S, Komisarenko S & Skok M (2015) α7 Nicotinic Acetylcholine Receptor-Specific Antibody Induces Inflammation and Amyloid β42 Accumulation in the Mouse Brain to Impair Memory. PLoS One. 2015;10(3):e0122706. doi:10.1371/journal.pone.0122706
Azam L, Papakyriakou A, Zouridakis M, Giastas P, Tzartos SJ, & McIntosh JM (2015) Molecular interaction of α-conotoxin RgIA with the rat α9 nAChR. Mol Pharmacol. 2015;87(5):855-864. doi:10.1124/mol.114.096511
Zouridakis M, Giastas P, Zarkadas E, Chroni-Tzartou D, Bregestovski P, & Tzartos SJ (2014) Crystal structures of free and antagonist-bound states of human α9 nicotinic receptor extracellular domain. Nat Struct Mol Biol.2014;21(11):976-980. doi:10.1038/nsmb.2900
Niarchos A, Zouridakis M, Douris V, Georgostathi A, Kalamida D, Sotiriadis A, Poulas K, Iatrou K, & Tzartos SJ (2013)Expression of a highly antigenic and native-like folded extracellular domain of the human α1 subunit of muscle nicotinic acetylcholine receptor, suitable for use in antigen specific therapies for Myasthenia Gravis. PLoS One.2013;8(12):e84791. doi:10.1371/journal.pone.0084791
Koval L, Lykhmus O, Kalashnyk O, Bachinskaya N, Kravtsova G, Soldatkina M, Zouridakis M, Stergiou C, Tzartos S,Tsetlin V, Komisarenko S, & Skok M (2011) The presence and origin of autoantibodies against α4 and α7 nicotinic acetylcholine receptors in the human blood: possible relevance to Αlzheimer's pathology. J Alzheimers Dis.2011;25(4):747-761. doi:10.3233/JAD-2011-101845
Lykhmus O, Koval L, Skok M, Zouridakis M, Zisimopoulou P, Tzartos SJ, Tsetlin V, Granon S, Changeux JP, Komisarenko S & Cloëz-Tayarani I (2011) Antibodies against extracellular domains of alpha4 and alpha7 subunitsalter the levels of nicotinic receptors in the mouse brain and affect memory: possible relevance to Alzheimer'spathology. J Alzheimers Dis. 2011;24(4):693-704. doi:10.3233/JAD-2011-101842
Lykhmus O, Koval L, Pavlovych S, Zouridakis M, Zisimopoulou P, Tzartos S, Tsetlin V, Volpina O, Cloëz-Tayarani I, Komisarenko S & Skok M (2010) Functional effects of antibodies against non-neuronal nicotinic acetylcholine receptors. Immunol Lett. 2010;128(1):68-73. doi:10.1016/j.imlet.2009.11.006
Zouridakis M, Zisimopoulou P, Poulas K & Tzartos SJ (2009) Recent advances in understanding nicotinic acetylcholine receptor structure. IUBMB Life. 2009;61(4):407-423. doi:10.1002/iub.170
Zouridakis M, Zisimopoulou P, Eliopoulos E, Poulas K & Tzartos SJ (2009) Design and expression of human α7 nicotinic acetylcholine receptor extracellular domain mutants with enhanced solubility and ligand-binding properties Biochim Biophys Acta. 2009;1794(2):355-366. doi:10.1016/j.bbapap.2008.11.002
Zouridakis M, Kostelidou K, Sotiriadis A, Stergiou C, Eliopoulos E, Poulas K & Tzartos SJ (2007) Circular dichroismstudies of extracellular domains of human nicotinic acetylcholine receptors provide an insight into their structure. Int J Biol Macromol. 2007;41(4):423-429. doi:10.1016/j.ijbiomac.2007.05.012
Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, Sideri A, Zouridakis M & Tzartos SJ. (2007) Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. FEBS J. 2007;274(15):3799-3845. doi:10.1111/j.1742-4658.2007.05935.x
Kostelidou K, Trakas N, Zouridakis M, Bitzopoulou K, Sotiriadis A, Gavra I & Tzartos SJ (2006) Expression and characterisation of soluble forms of the extracellular domains of the β, γ and ε subunits of the human muscle acetylcholine receptor. FEBS J. 2006;273(15):3557-3568. doi:10.1111/j.1742-4658.2006.05363.x





